BestaMed was appreciated with excellent results by the Bulgarian Medicines Verification Organisation during the on-site test on August 24, 2018.
One of the first functioning systems for the verification of medicinal products in the wholesale trade has passed a successful pilot testing. This is the system implemented by BestaMed – the largest provider of parallel import medicines and one of the progressively growing distributors on the territory of Bulgaria. The purpose of the verification is to protect patients from falsified medicines and to ensure the safety of drug delivery throughout the chain.
Falsified medicines are a growing threat worldwide. To keep them out of the legal distribution chain the pharmaceutical sector has joined forces in developing a verification system that will be able to check whether medicinal products are genuine. The system is intended to comply with the EU’s new requirements for preventing falsified medicinal products and to ensure that patients have authentic medicines.
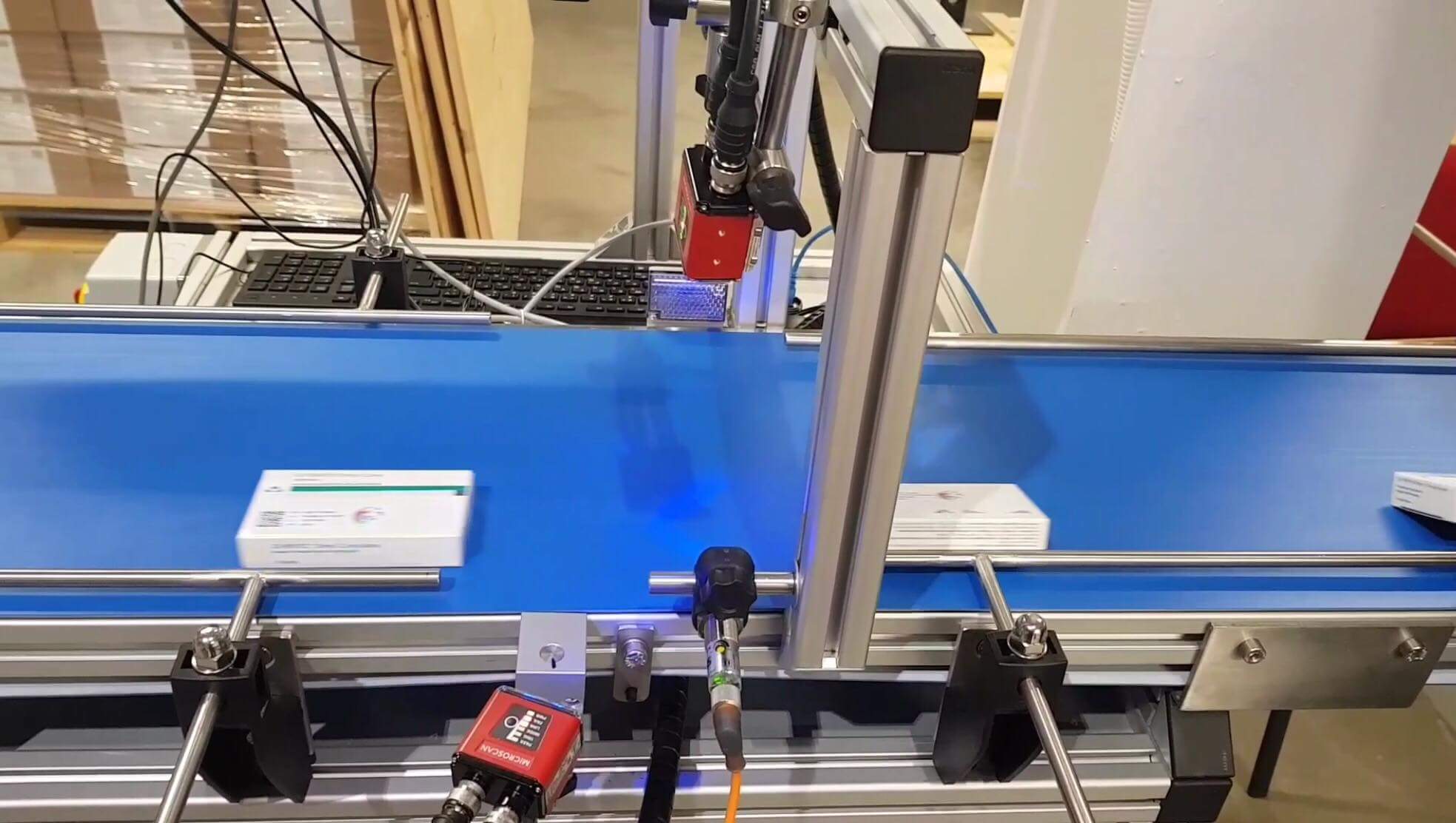
The pilot testing at BestaMed was carried out on 24 August 2018 by the Bulgarian Drug Enforcement Organization. The team was composed of the Executive Director and Quality Manager of BgMVO, Mrs. Iliana Paunova and Mr. Nikolay Tsvetanov from BgMVO team. They visited BestaMed logistics center, where the verification system has been implemented since the beginning of July as part of the pilot program to implement the EU Counterfeit Drug Directive in the European Union.
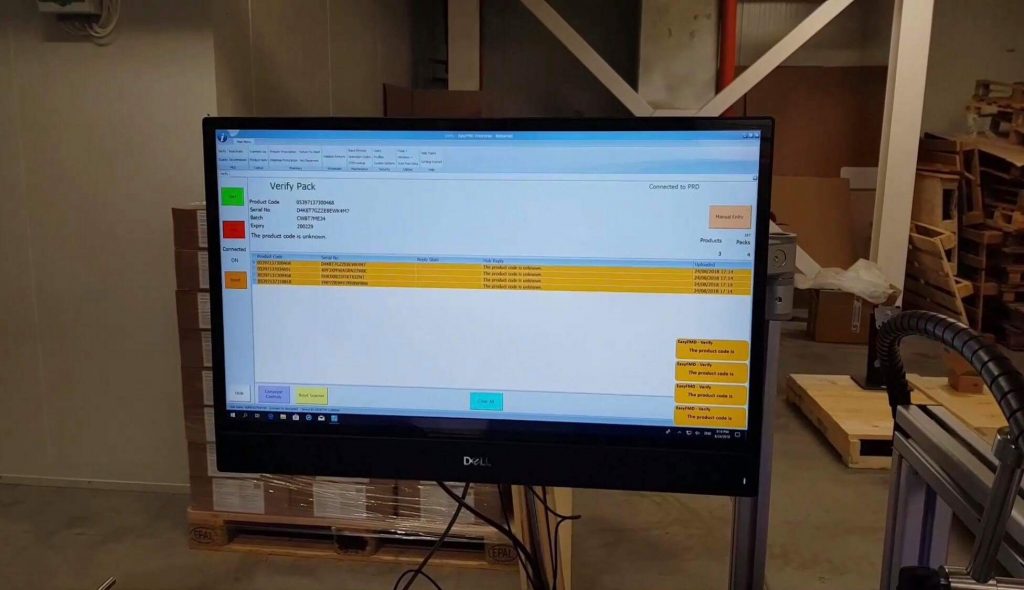
BgMVO performed on-the-spot all verification scenarios to assess that the system correctly takes into account the different assumptions of a comprehensive verification of the origin of the medicinal products. Pilot testing results have shown that BestaMed’s system works flawlessly in each of the tested scenarios. The overall assesment of BgMVO was excellent. The IT verification provider of Bestamed is QPS – Quick Pharma Solutions, the solution name is Easy FMD.
BestaMed provides strategic solutions and services to companies that are operating in the Bulgarian and European pharmaceutical sector with expertise in the field of import, distribution and pre-distribution of original medicinal products in Bulgaria and the European Union. BestaMed is a co-founder and member of BAMPTD – Bulgarian Association for Medicines Parallel Trade Development a member of the EAEPC – European Association of Euro-pharmaceutical Companies.
Bulgarian Medicines Verification Organisation was established on 14 March 2016 in Sofia as a non-profit association to support the implementation in Bulgaria of Directive 2011/62/EU for preventing entry of falsified medicinal products into the legal supply chain.